by Iain D Miller, PhD, MBA Abridged version; full article available here
Clinical diagnostic technology is the backbone of precision medicine and the foundation on which the NHS “Fit for the Future” plan rests. Pathology testing alone underpins 95% of care pathways, while diagnostics guide 70% of all healthcare decisions. The market reflects this: the global in-vitro diagnostics sector is worth more than $100 billion, with the UK’s £3.5 billion share already a pillar of the economy. But these figures are only scratching the surface of the potential boon precision care could bring to the UK economy, for which a value of £58bn has been estimated.
 Despite the potential value that diagnostic technology brings to the NHS and the economy, the pipeline of innovation is dangerously underfunded. Two of the NHS’s three future-focused missions — early diagnosis/prevention, and the decentralisation of care — are explicitly predicated on testing and digital diagnostics. But without capital to scale, those ambitions will falter. The technology exists, the need is undeniable, and the economic upside is substantial. What’s missing is the venture capital confidence to back diagnostics with the same fervour once reserved for fintech or consumer apps.
Despite the potential value that diagnostic technology brings to the NHS and the economy, the pipeline of innovation is dangerously underfunded. Two of the NHS’s three future-focused missions — early diagnosis/prevention, and the decentralisation of care — are explicitly predicated on testing and digital diagnostics. But without capital to scale, those ambitions will falter. The technology exists, the need is undeniable, and the economic upside is substantial. What’s missing is the venture capital confidence to back diagnostics with the same fervour once reserved for fintech or consumer apps.
While the VC sector is robust, with UK life science raises totalling £1.2Bn in the first half of 2025, the inconvenient truth is that investors are generally reluctant to support innovative new early-stage diagnostic ventures. Instead, they are increasingly favouring follow-on (insider) rounds, which reached a historical high of 46% of all seed rounds in Q2, 2025.
Innovators must realise that, in addition to the usual foundational investor diligence criteria (IP, team, market, unmet need, USP, etc), investors (both venture and corporate) will seek solid evidence of clinical validity (rarely investing before TRL 4 to retire some of the high validation risk mentioned earlier) and a near term path to commercial validation within 2-3 years. Angels and Seed Enterprise Investment Scheme (SEIS) Funds maybe more accessible but offer only modest support.
The hard reality, and catch-22 for diagnostic innovators, is that funding is required to make a venture investible. In practice, these financial constraints mean that you will have to proceed one small step at a time in the early stages, rather than via a single moonshot. In practice, diagnostic innovators must both pursue an alternative, incremental, early-stage funding mix and develop a strategy to accelerate time to first sale.
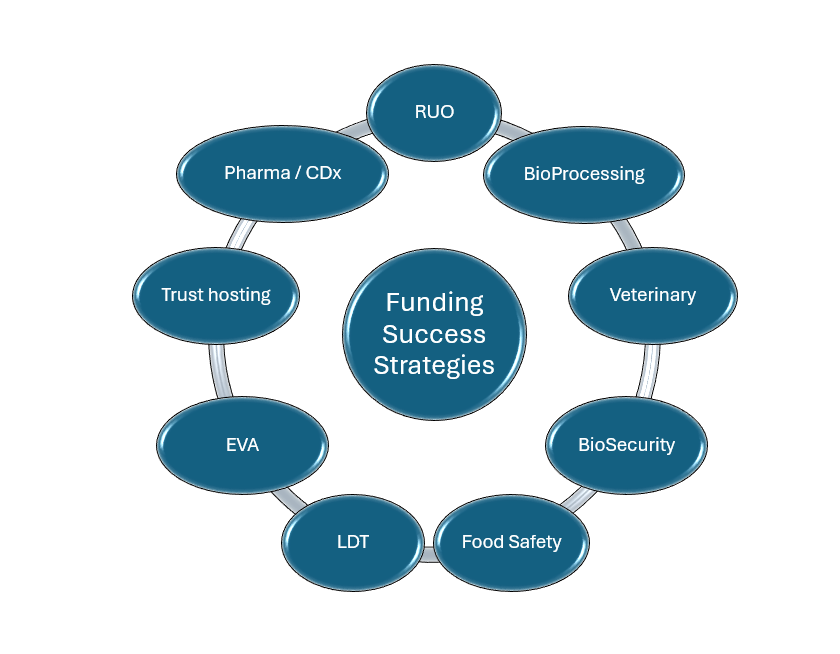
 In the full version of this article, I describe some possible sources for early-stage incremental funding. I also detail 12 strategies to reduce evidentiary requirements, and accelerate commercial validation, thereby enhancing investibility. These strategies, illustrated schematically here, fall into 3 broad approaches:
In the full version of this article, I describe some possible sources for early-stage incremental funding. I also detail 12 strategies to reduce evidentiary requirements, and accelerate commercial validation, thereby enhancing investibility. These strategies, illustrated schematically here, fall into 3 broad approaches:
- Seek initial commercial validation of non-clinical products
- Build a veterinary portfolio
- Accelerate clinical product development via service offerings, embedded co-development, and/or conditional access mechanisms
About the Author
 Iain Miller is a MedTech sector veteran with over 30 years of diverse executive-level experience, most recently at C-suite & Board level. He has led 4 medtech SMEs and sat on 6 Boards. His career focus has been on In-Vitro Diagnostic and Precision Medicine sectors. He has held senior leadership roles in diverse international SMEs, academic medical centres, venture-backed & listed blue-chip companies including GE Medical Diagnostics, Biomerieux & Massachusetts General Hospital. He also sat on a NICE technology Assessment panel from 2014-17. He currently serves as a Board member of Virax Biolabs (NASDAQ: VRAX). Previously, from 2019-2025, he served as founding CEO of Presymptom Health, a developer of AI-powered blood tests for infection and, from 2022-2025, as Non-Executive Director of Pictura Bio, an Oxford-based developer of rapid AI-powered pathogen detection tests. Iain has extensive US market experience (20 years based in Boston) and holds dual US/UK citizenship. He has a PhD in Biomedical Engineering from Strathclyde University and an MBA from Edinburgh Business School.
Iain Miller is a MedTech sector veteran with over 30 years of diverse executive-level experience, most recently at C-suite & Board level. He has led 4 medtech SMEs and sat on 6 Boards. His career focus has been on In-Vitro Diagnostic and Precision Medicine sectors. He has held senior leadership roles in diverse international SMEs, academic medical centres, venture-backed & listed blue-chip companies including GE Medical Diagnostics, Biomerieux & Massachusetts General Hospital. He also sat on a NICE technology Assessment panel from 2014-17. He currently serves as a Board member of Virax Biolabs (NASDAQ: VRAX). Previously, from 2019-2025, he served as founding CEO of Presymptom Health, a developer of AI-powered blood tests for infection and, from 2022-2025, as Non-Executive Director of Pictura Bio, an Oxford-based developer of rapid AI-powered pathogen detection tests. Iain has extensive US market experience (20 years based in Boston) and holds dual US/UK citizenship. He has a PhD in Biomedical Engineering from Strathclyde University and an MBA from Edinburgh Business School.